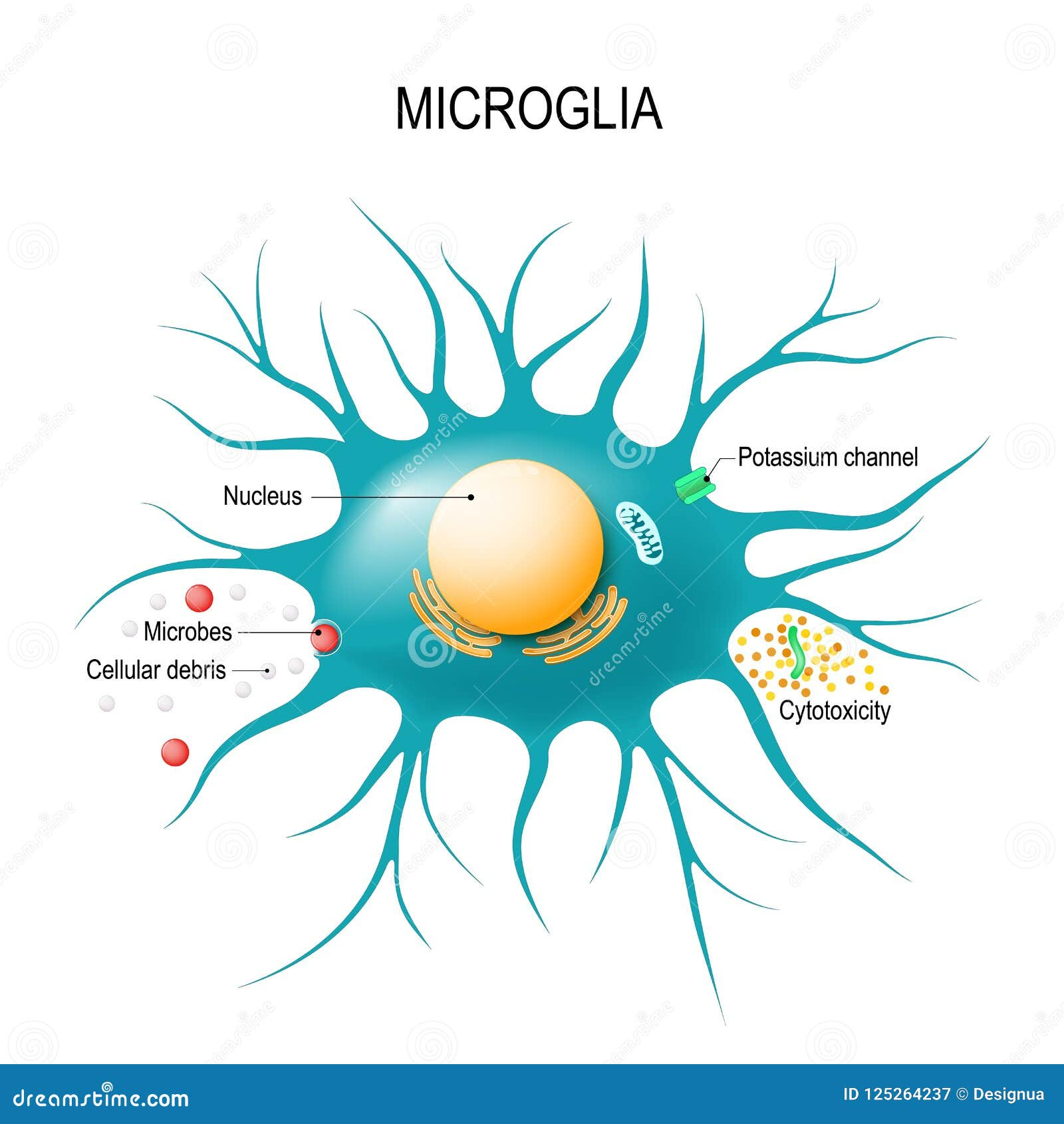
Microglial cells play a crucial role in the brain’s immune system, acting as vigilant sentinels that protect against neurodegenerative diseases such as Alzheimer’s. These specialized immune cells monitor the brain for signs of injury or illness, helping to remove damaged cells and facilitating synaptic pruning to maintain neural network efficiency. This process, while essential for healthy brain function, can sometimes spiral into harmful aberrations, contributing to the onset of conditions like Alzheimer’s and Huntington’s disease. Pioneering research led by Beth Stevens at Boston Children’s Hospital has illuminated the link between dysfunctional microglial activity and neurodegeneration, paving the way for innovative biomarkers and treatment strategies. The growing understanding of microglial cells opens new avenues for therapeutic interventions aimed at improving the lives of millions affected by Alzheimer’s and related disorders.
Often referred to as the brain’s immune defenders, microglial cells play an instrumental role in maintaining neurological health by constantly surveying the central nervous system. Their proactive engagement in the clearance of cellular debris and their participation in synaptic remodeling are vital processes that ensure efficient communication between neurons. However, when these cells become dysregulated, they can inadvertently contribute to the progression of neurodegenerative disorders such as Alzheimer’s disease. Pioneering insights from researchers like Beth Stevens bring to light the significance of these cells in the context of neurodegeneration, highlighting how their activity can either protect or harm brain function. Understanding the dual roles of these immune cells is essential for developing strategies that could mitigate the impact of neurodegenerative diseases.
Understanding Microglial Cells and Their Role in Alzheimer’s Research
Microglial cells play a crucial role in maintaining brain health, acting as the brain’s immune system. These specialized cells are responsible for identifying and eliminating cellular debris, damaged neurons, and pathogens. Their function includes synaptic pruning, a vital process that shapes neural circuits during development, ensuring that signals between neurons are efficient and relevant. In the context of Alzheimer’s disease, research led by scientists like Beth Stevens has highlighted the importance of microglia in neurodegenerative processes. Abnormalities in microglial activity can lead to excessive synaptic pruning, contributing to cognitive decline and the progression of Alzheimer’s.
As research in Alzheimer’s continues to evolve, understanding the dynamics of microglial cells is paramount. These cells not only respond to damage but also participate in signaling pathways that can either foster brain health or facilitate neurodegeneration. The groundbreaking findings from Stevens’ lab at Boston Children’s Hospital have paved the way for new therapeutic strategies aimed at modulating microglial response. By targeting these immune cells, there is potential to alter disease progression, offering hope for the millions affected by Alzheimer’s and other neurodegenerative diseases.
The Impact of Aberrant Synaptic Pruning on Neurodegenerative Diseases
Synaptic pruning, a normal developmental process, can become maladaptive in the context of neurodegenerative diseases. Research suggests that when microglial cells mismanage this process, it can lead to significant consequences, including synaptic loss and cognitive impairment in conditions like Alzheimer’s and Huntington’s disease. The Stevens Lab has shown that aberrant pruning not only accelerates neural degeneration but also disrupts communication between neurons, exacerbating the symptoms of these disorders. Such insights emphasize the dual role of microglia in both protecting and harming the brain, depending on their state and activity.
Furthermore, identifying aberrant synaptic pruning as a key factor in neurodegeneration opens new avenues for therapeutic intervention. Drugs designed to fine-tune microglial activity and promote proper synaptic maintenance could potentially halt or even reverse some cognitive decline. As our understanding of these processes deepens, it becomes increasingly clear that the brain’s immune system is a vital target for Alzheimer’s research. Scientists, including Stevens, are optimistic about developing strategies that could ultimately lead to better management and treatment options for those suffering from neurodegenerative diseases.
Neurodegenerative Diseases: The Role of the Brain’s Immune System
Neurodegenerative diseases, such as Alzheimer’s, are characterized by progressive degeneration of neuronal cells, leading to loss of cognitive function and, ultimately, death. An increasing body of research underscores the pivotal role of the brain’s immune system, particularly microglial cells, in these conditions. Microglia not only provide immune defense but also modulate brain homeostasis and inflammation. Dysregulation in microglial functions can lead to chronic inflammation, which is linked to the progression of various neurodegenerative diseases.
Beth Stevens’ research has elaborated on the relationship between neuroinflammation and neurodegeneration, suggesting that targeted manipulation of microglial activity could mitigate these diseases. By enhancing the brain’s immune responses or correcting the pruning processes, it may be possible to protect neuronal integrity and preserve synaptic function. This perspective highlights the importance of ongoing Alzheimer’s research in uncovering how the brain’s immune system can be harnessed to combat the effects of neurodegenerative diseases.
Innovative Biomarkers and Treatment Strategies in Alzheimer’s Disease
The emergence of innovative biomarkers is transforming Alzheimer’s research and treatment approaches. Beth Stevens has been influential in identifying characteristics of microglial activity that could serve as early indicators of neurodegenerative disease onset. By focusing on how microglia change in response to neurodegenerative processes, researchers can develop more effective diagnostic tools and treatment strategies. These biomarkers could help in identifying individuals at risk for Alzheimer’s before significant cognitive decline occurs.
Furthermore, advancements in understanding the molecular mechanisms behind microglial dysfunction are paving the way for new treatments. By targeting the specific pathways involved in microglial activation and synaptic pruning, researchers can create therapies that enhance brain health. These strategies hold promise for improving the quality of life for those affected by Alzheimer’s as well as potentially slowing the progression of the disease.
The Contribution of Basic Science in Alzheimer’s Research
The journey of scientific discovery is often driven by fundamental research that may not have immediate clinical implications. Beth Stevens emphasizes the value of curiosity-driven science, which has led to revolutionary findings in Alzheimer’s research. The foundational studies focused on microglial biology have revealed critical insights into how the brain manages homeostasis and responds to injury. These studies exemplify how basic research fosters innovation, leading to new concepts that translate into therapeutic strategies against Alzheimer’s and other neurodegenerative diseases.
Funding from agencies like the National Institutes of Health (NIH) has been essential in enabling investigators like Stevens to pursue questions about the brain’s immune responses and their implications for diseases like Alzheimer’s. Such investment in basic science underlines the importance of nurturing exploratory research that can lead to breakthroughs in understanding complex diseases. Without these foundational studies, the significant advances achieved in the field of Alzheimer’s would likely not have been possible.
Beth Stevens: A Pioneer in Neuroimmunology
Beth Stevens has emerged as a pioneering figure in neuroimmunology, particularly in understanding the roles of microglial cells in the brain. Her groundbreaking work has reshaped the way the medical community perceives microglia, not just as passive defenders of the brain but as active participants in neural development and degeneration. Stevens’ research highlights the dual nature of microglia, which can support synaptic health while also contributing to neurodegenerative processes when mismanaged.
Recognized for her contributions, Stevens has received numerous accolades, including the prestigious MacArthur Fellowship. Her work serves as a model for how neurobiology and immunology intersect, providing a richer understanding of brain health and diseases. By elucidating the complex behaviors of microglial cells in Alzheimer’s, Stevens is not only advancing academic knowledge but also pushing forward the potential for innovative treatments.
Exploring the Synaptic Functions of Microglial Cells
Microglial cells play a vital role in synaptic functions that are crucial for neural communication. They actively participate in synaptic pruning during development, ensuring that the brain’s neural networks are efficiently organized. This synaptic sculpting is essential for learning and memory, making microglia key players in cognitive function. However, when microglial cells become dysfunctional, the balance of synaptic pruning can be lost, potentially leading to conditions like Alzheimer’s disease.
Research, particularly at the hands of scientists like Beth Stevens, has illustrated how alterations in microglial activity can result in an increased loss of synapses, correlating with symptoms of neurodegeneration. Understanding the mechanisms behind this mismanagement is essential for developing therapeutic interventions aimed at restoring the healthy function of microglial cells, thereby protecting synaptic integrity and supporting cognitive health.
The Future of Alzheimer’s Research: Insights from Microglial Studies
The future of Alzheimer’s research is poised for significant advancements thanks to the insights gained from studying microglial cells. As researchers deepen their understanding of the role these immune cells play in neurodegenerative diseases, new avenues for prevention, diagnosis, and treatment are expected to emerge. Studies that focus on the interplay between microglial function and synaptic health may unveil critical pathways that could be targeted to prevent or slow disease progression.
Additionally, the development of therapeutic strategies that enhance the beneficial functions of microglial cells while inhibiting their detrimental effects holds promise for effective treatments. As we continue to explore these fascinating cells, the potential to fundamentally change the landscape of Alzheimer’s care becomes more tangible. This interplay between basic research and translational efforts highlights the critical importance of supporting scientific inquiry as we search for solutions to Alzheimer’s and related disorders.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are crucial in Alzheimer’s research as they act as the brain’s immune system. They patrol the brain for inflammation and damage, clearing dead cells and participating in synaptic pruning. Aberrant microglial activity has been linked to the progression of Alzheimer’s disease, providing insights for new treatments.
How do microglial cells relate to neurodegenerative diseases?
Microglial cells are implicated in various neurodegenerative diseases, including Alzheimer’s and Huntington’s. Their primary function is to maintain brain health, but dysfunctional microglia can lead to reduced synaptic pruning, promoting neuroinflammation and exacerbating disease pathology.
What is the significance of synaptic pruning by microglial cells in brain health?
Synaptic pruning by microglial cells is essential for healthy brain development and function. It helps refine neural circuits by removing excess synapses, but when this process goes awry, it can contribute to neurodegenerative diseases like Alzheimer’s, highlighting the delicate balance microglia maintain in synaptic health.
How has Beth Stevens’ research changed the understanding of microglial cells?
Beth Stevens’ research has transformed the understanding of microglial cells by demonstrating their dual role in brain immunity and synaptic pruning. Her findings reveal that dysfunctional microglia contribute to the pathology of Alzheimer’s and other neurodegenerative diseases, opening new avenues for treatment.
What potential do microglial cells hold for developing new Alzheimer’s treatments?
Microglial cells represent a promising target for developing new Alzheimer’s treatments. Understanding their role in synaptic pruning and neuroinflammation can lead to the identification of biomarkers and therapeutic strategies aimed at modulating microglial activity to slow disease progression.
Why are microglial cells considered important for the brain’s immune system?
Microglial cells are considered vital for the brain’s immune system because they monitor the central nervous system for injury and infection, respond to harmful stimuli, clear cellular debris, and assist in synaptic pruning, thus maintaining overall brain health and function.
How do microglial cells contribute to synaptic pruning during brain development?
During brain development, microglial cells contribute to synaptic pruning by selectively removing weaker or excess synapses based on activity levels. This process ensures that the neural circuits are efficiently wired for optimal brain function, a critical aspect that if disrupted can lead to neurodegenerative conditions.
| Key Points |
|---|
| Microglial cells act as the brain’s immune system, protecting the brain from illness and injury. |
| Beth Stevens has transformed the understanding of microglial cells in relation to Alzheimer’s and other disorders. |
| Aberrant pruning by microglia can contribute to neurodegenerative diseases like Alzheimer’s and Huntington’s. |
| Research in Stevens’s lab has led to potential new biomarkers and treatments for Alzheimer’s disease. |
| The work highlights the importance of basic science, which often leads to unexpected discoveries related to disease. |
Summary
Microglial cells play a crucial role in the brain’s immune response. They are essential for maintaining healthy brain function by clearing damaged cells and supporting neuronal connections. Research led by Beth Stevens has uncovered important links between microglial activity and neurodegenerative diseases like Alzheimer’s. This groundbreaking work may pave the way for new diagnostic tools and therapies, significantly impacting the lives of millions affected by these conditions. By understanding and harnessing the power of microglial cells, we can aim for better outcomes in the fight against Alzheimer’s disease.