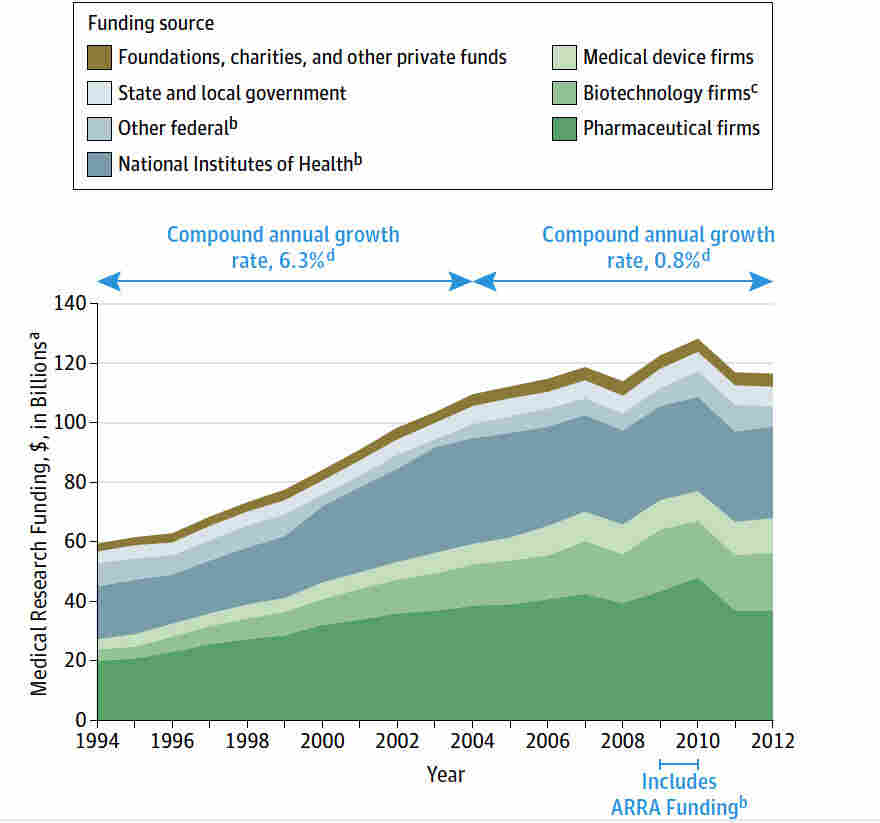
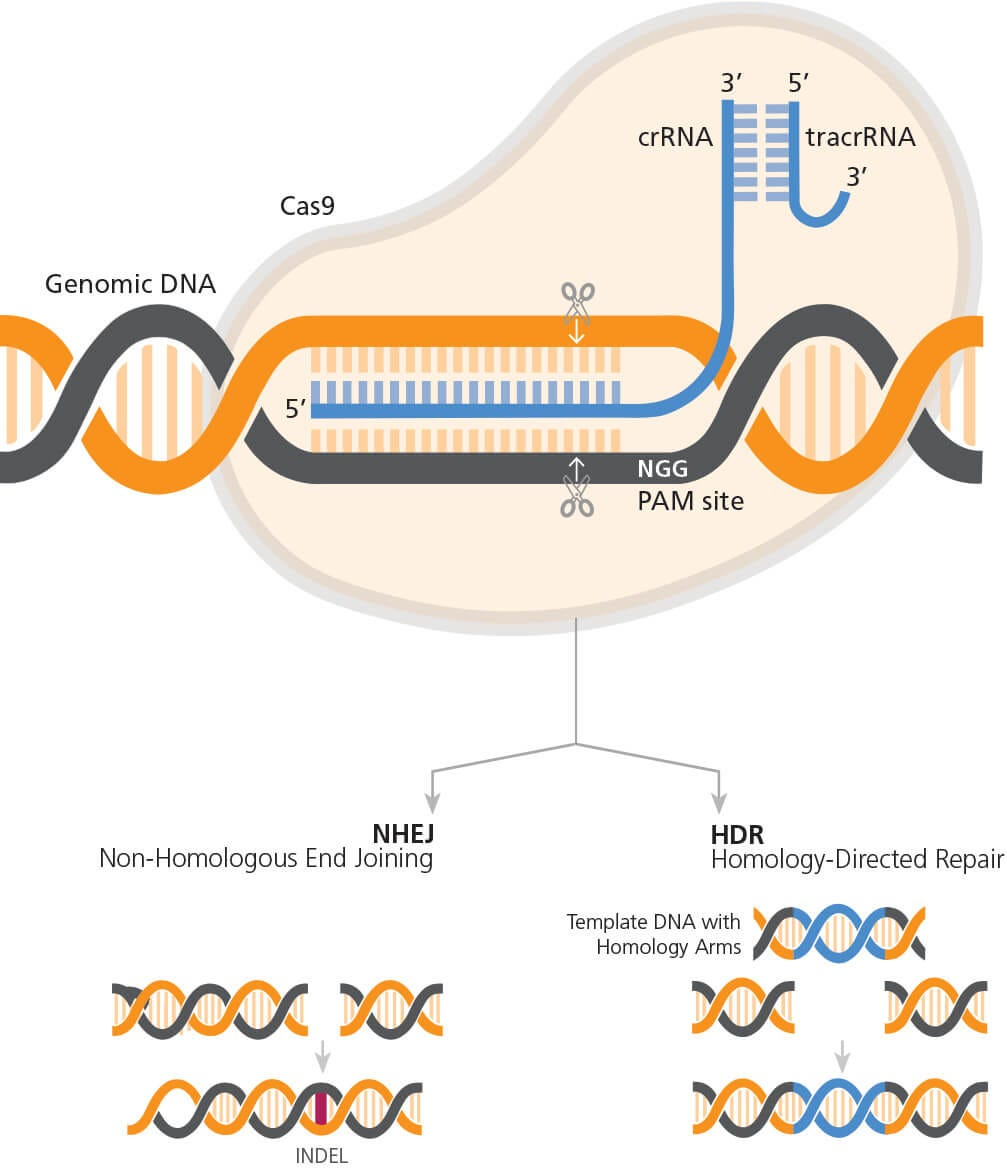
Medical research funding is vital for advancing healthcare and ensuring patient safety in research. Without adequate financial support, critical projects face interruptions that disrupt the oversight required to protect participants in clinical trials. The recent funding cuts threaten not only the integrity of research but also the ethical standards maintained through IRB oversight, which is essential for safeguarding participant rights. As organizations await essential NIH research grants, the impact of funding cuts reverberated through institutions, raising concerns about clinical trial ethics and the potential consequences for patient safety. Addressing these challenges is crucial to maintain public confidence in medical research and its role in promoting health innovations.
The financial backing for healthcare studies plays a pivotal role in advancing scientific knowledge and ensuring the well-being of participants. Budget constraints can severely hinder research efforts, leading to ethical dilemmas regarding patient safety and the necessary oversight provided by institutional review boards (IRBs). In the landscape of medical experimentation, the lack of support could derail promising clinical trials, fueling skepticism among the public and jeopardizing trust in the research community. Meanwhile, the absence of vital federal funds, such as those from the National Institutes of Health (NIH), reinforces the importance of collaborative research commitments to uphold clinical trial integrity. This delicate balance underscores the pressing need for sustainable investment in medical research to foster innovation and protect human subjects.
The Consequences of Funding Cuts on Medical Research
The halt in funding can have devastating effects on medical research, particularly in enhancing patient safety. When funding is cut, it restricts the resources needed for comprehensive Institutional Review Board (IRB) oversight, which is crucial for maintaining ethical standards and ensuring patient welfare. Projects previously supported by substantial grants face immediate disruption, leaving many studies at risk of halting entirely or being unable to initiate new sites. This not only jeopardizes research outcomes but also raises concerns about the ethical implications of abandoning ongoing trials that directly affect volunteer participants.
Furthermore, the cancellation of NIH research grants is particularly alarming as these funds play a pivotal role in empowering institutions to prioritize patient safety. The absence of adequate financial resources limits investigators’ ability to implement necessary infrastructure for monitoring research protocols. Without proper oversight, there’s a real danger of encroaching on participants’ rights and welfare, which could perpetuate mistrust in the research community and hinder future participatory efforts.
The Role of Medical Research Funding in Patient Safety
Medical research funding is vital in ensuring patient safety through the sustained operation of IRBs, which are responsible for rigorous evaluation and approval of research studies. These funds allow IRBs to operate efficiently, enabling them to assess the risks involved in various study designs and help researchers mitigate potential dangers to participants. As the quality of oversight diminishes due to a lack of financial support, the likelihood of risking patient safety escalates, highlighting the intrinsic link between funding and the ethical conduct of research.
Additionally, significant funding allows for ongoing training programs for research staff and IRB members, ensuring they remain updated about best practices and evolving ethics related to patient safety. Cutting these funds might lead to a less informed workforce, compromising the ethical standards upheld by the IRB process. In turn, this could have a cascading effect on the overall integrity of clinical trials and ultimately, the quality of care patients receive in medical settings.
IRB Oversight: A Critical Component of Ethical Research
Institutional Review Boards (IRBs) act as a safeguard against ethical violations in medical research, ensuring that the rights and welfare of research participants are prioritized. The recently implemented requirement for studies to undergo single IRB review for multisite studies underscores the growing recognition of streamlined oversight to protect patient safety. However, this system heavily relies on consistent funding to maintain thorough evaluations and ongoing support for research teams.
Without adequate funding, the IRBs face significant challenges in delivering the comprehensive oversight necessary to uphold ethical standards. This gap can leave participants vulnerable as research protocols may not be thoroughly vetted for potential risks. Maintaining the integrity of the IRB process is essential, and funding cuts threaten not only the immediate safety of participating individuals but also damage the public’s trust in medical research as a whole.
The Historical Context of Oversight in Medical Research
The historical precedents underscore the necessity for stringent oversight in medical research. Events such as the Tuskegee Study and various unethical experiments conducted during World War II catalyzed the establishment of IRBs as a response to protect participants and ensure informed consent. These historical lessons highlight the importance of maintaining rigorous ethical standards within medical research protocols, which are supported through adequate funding.
Funding cutbacks threaten to undermine the progress made from these historical injustices by potentially compromising the ethical oversight systems that have been put in place. Ensuring that IRBs can effectively monitor research studies is essential not only for compliance with ethical guidelines but also for upholding the trust that has been painstakingly rebuilt over decades. The ongoing education and training of IRB personnel funded by research grants are critical for preventing a regression in ethical standards established in the wake of past abuses.
Innovations at Risk: The Impact of Stalled Funding
Funding interruptions not only halt ongoing research but also stifle innovation in the medical field. Collaborative research efforts often depend on multiple institutions working together. When federal grants are frozen or cut, as seen with the recent stop-work orders, the momentum necessary for transformative discoveries is jeopardized. The delay or cessation of studies can adversely affect patients who rely on breakthroughs in treatment and therapies.
Moreover, stalled funding disrupts networks of collaboration that have been instrumental in breaking down barriers to innovation. For instance, the SMART IRB system was developed to streamline the approval process for multisite research, reducing bureaucratic delays that could stifle scientific progress. However, without the necessary funds, such systems become inoperative at a time when efficient collaboration is crucial to addressing urgent health challenges.
The Collective Efforts to Safeguard Patient Welfare
The collective efforts of healthcare professionals, ethics boards, and research institutions converge toward one primary goal: the protection of patient welfare in clinical trials. Patient safety must be at the forefront of research initiatives, ensuring that individuals who volunteer for studies are treated with the utmost respect. However, these efforts rely synergistically on adequate funding to sustain their crucial operations.
Increased investment in medical research funding not only strengthens institutional capacities but also fosters a culture of safety and ethical rigor. Training opportunities provided through grants enhance IRB capabilities, enabling them to effectively assess studies and communicate transparently with potential participants. This comprehensive approach ensures a commitment to protecting patients throughout the research process.
Implications of Research Funding on Public Trust
Research funding plays a critical role in shaping public perceptions of medical research and patient safety. The confidence people place in clinical trials is directly tied to how transparently research is conducted, overseen, and funded. Any disruption caused by funding cuts can lead to public skepticism, raising concerns about the motivations behind research activities and their potential impacts on participant welfare.
When funding streams are threatened, the potential for positive community engagement decreases significantly. Engaging with community members about ongoing research, ensuring their voices are heard, and addressing their concerns depends heavily on the resources available for outreach efforts. A commitment to funding not only enhances the quality of research but also fortifies public trust in the healthcare system, thus fostering a collaborative environment conducive to ongoing studies.
Balancing Ethics and Innovation in Medical Research
As the dynamics of medical research evolve, striking a balance between innovation and ethical oversight becomes increasingly crucial. The ethical frameworks established by IRBs must be adaptable to the fast-paced nature of scientific advancements while ensuring the protection of participants. Nonetheless, balancing these demands necessitates substantial funding that supports both ethical compliance and pioneering research.
Innovative research that seeks to explore uncharted territories in medicine requires the kind of rigorous oversight that has historically proven essential to patient safety. Capital investments facilitate the necessary infrastructure that equips research boards to maintain this balance, assuring that patients can partake in groundbreaking studies with confidence. The relationship between funding, ethics, and innovation is not just beneficial; it is a vital component of responsible medical progress.
The Future of Medical Research Without Sufficient Funding
Looking to the future, the implications of declining research funding resonate deep within the medical community. Without sufficient financial support, researchers may face difficult choices about which studies to pursue, potentially sidelining important investigative work. The overall trajectory of medical advancements becomes tenuous in an environment where funding cuts stifle ambitious research initiatives.
Furthermore, the ripple effects of diminished funding extend far beyond research institutions. The disruption of clinical trials may compromise patient access to new treatments, leading to stagnation in healthcare advancements. These considerations highlight the need for policymakers and stakeholders to advocate for robust support for medical research funding to safeguard the interests of patients and the integrity of the research community.
Frequently Asked Questions
How do funding cuts impact patient safety in medical research?
Funding cuts, such as the recent halt of $2 billion in federal research grants, significantly hinder efforts to protect patient safety in medical research. The lack of financial resources disrupts the functioning of Institutional Review Boards (IRBs), which are critical for monitoring and ensuring that research complies with ethical standards. Without sufficient funding, IRBs cannot effectively oversee clinical trials, leading to potential risks for participants and deteriorating public trust in research.
What are NIH research grants and how do they promote ethical oversight in clinical trials?
NIH research grants provide essential funding for medical studies involving human participants, ensuring compliance with ethical standards through rigorous oversight by IRBs. This funding supports the necessary structure for monitoring clinical trials, assessing risks, and protecting participant welfare. By ensuring that these funds are available, researchers can conduct safety-oriented studies that align with ethical regulations.
What role do IRB oversight and funding play in maintaining ethics in clinical trials?
IRB oversight is crucial for maintaining ethics in clinical trials, as IRBs review and approve research proposals to ensure the safety and rights of participants. Adequate funding allows IRBs to operate effectively, review proposals thoroughly, and provide necessary trainings for researchers. Cuts in funding can jeopardize these processes, increasing risks for participants involved in medical research.
Is there a relationship between the impact of funding cuts and research participant trust?
Yes, the impact of funding cuts is directly related to research participant trust. When funding is cut, IRB oversight may be compromised, leading to ethical concerns in clinical trials. This scenario can harm the relationship between researchers and participants, fostering skepticism and mistrust towards medical research institutions, which are vital for successful clinical trials.
What is the significance of the SMART IRB system in relation to NIH funding and patient safety?
The SMART IRB system streamlines the review process for multi-site clinical trials, enhancing patient safety by centralizing ethical oversight. Funding for this system allows for the seamless execution of significant collaborative research, which is critical for advancing medical innovations. Funding interruptions pose risks to both patient safety and the efficiency of this oversight system.
How does the cancellation of NIH research grants affect clinical trial ethics?
The cancellation of NIH research grants diminishes the resources available for ethical oversight in clinical trials. With fewer funds, the ability of IRBs to conduct thorough reviews, support investigators, and protect participant safety is compromised. This deterioration can lead to unethical practices in research, ultimately endangering the welfare of participants involved in clinical studies.
In what ways does insufficient funding challenge the oversight of clinical trials?
Insufficient funding challenges the oversight of clinical trials by limiting the resources available to IRBs, which are responsible for ensuring that research adheres to ethical standards. This can impede the recruitment of new sites, delay studies, and create barriers to adequate participant protection. Consequently, challenges in funding directly affect the overall integrity of medical research.
How do funding cuts threaten the integrity of medical research operations?
Funding cuts threaten the integrity of medical research operations by disrupting the essential processes of IRB oversight and ethical reviews. A weakened IRB system may lead to increased risks for study participants and undermine public confidence in medical research, ultimately slowing down scientific progress and innovation in healthcare.
| Key Points | Details |
|---|---|
| Funding Cuts Impact | A freeze of over $2 billion in federal research grants has disrupted multiple medical research areas at Harvard. |
| Importance of IRBs | Institutional Review Boards (IRBs) protect participants by ensuring compliance with laws and overseeing research proposals. |
| Reduction in Research Collaboration | The SMART IRB system facilitates multi-site research; funding cuts have halted new collaborations and delayed ongoing studies. |
| Historical Context | Past ethical breaches in research underscore the necessity for robust oversight and informed consent processes. |
| Broader Implications | Continuing funding cuts threaten public trust in medical research and can negatively impact patient safety and ethical standards. |
Summary
Medical research funding is crucial for ensuring patient safety and ethical oversight in clinical studies. The recent suspension of over $2 billion in federal research grants has significantly harmed collaborative efforts, notably impacting researchers’ ability to safeguard patients’ rights and welfare. The role of Institutional Review Boards (IRBs) in protecting study participants is now jeopardized, which could lead to delays and increased risks in ongoing research. This situation highlights the necessity of sustained financial support for medical research to uphold the integrity of clinical trials and maintain public trust.