In a groundbreaking shift for Alzheimer’s treatment, TIM-3 therapy for Alzheimer’s emerges as a promising approach leveraging the body’s immune response. Recent research indicates that by targeting the TIM-3 checkpoint molecule, it may be possible to unleash the brain’s microglia to combat the harmful amyloid plaques associated with the disease. This innovative therapy seeks to boost cognitive function in Alzheimer’s patients, a goal that has eluded many traditional treatments. With a focus on the TIM-3 role in Alzheimer’s pathology, scientists are optimistic about the potential of immune checkpoint therapy to enhance memory restoration. As we delve into the mechanisms behind microglia and Alzheimer’s, the promise of TIM-3 therapy highlights a new frontier in combating cognitive decline.
As scientists explore novel avenues for addressing cognitive decline in Alzheimer’s patients, TIM-3 therapy, also referred to as immune checkpoint therapy, stands out as a pivotal development. This strategy involves modulating the immune system to facilitate the action of microglia, the brain’s resident immune cells, enabling them to effectively clear amyloid-beta plaques that contribute to neurodegeneration. The implications of TIM-3 in late-onset Alzheimer’s and its role as an inhibitory molecule present a fascinating intersection between immunology and neurology. By enhancing the functionality of microglia, researchers are hopeful that TIM-3 therapy can lead to significant breakthroughs in Alzheimer’s treatment. As the understanding of microglia and Alzheimer’s deepens, so does the potential for innovative therapies to restore cognitive functions in affected individuals.
Understanding TIM-3 and Its Implications for Alzheimer’s Treatment
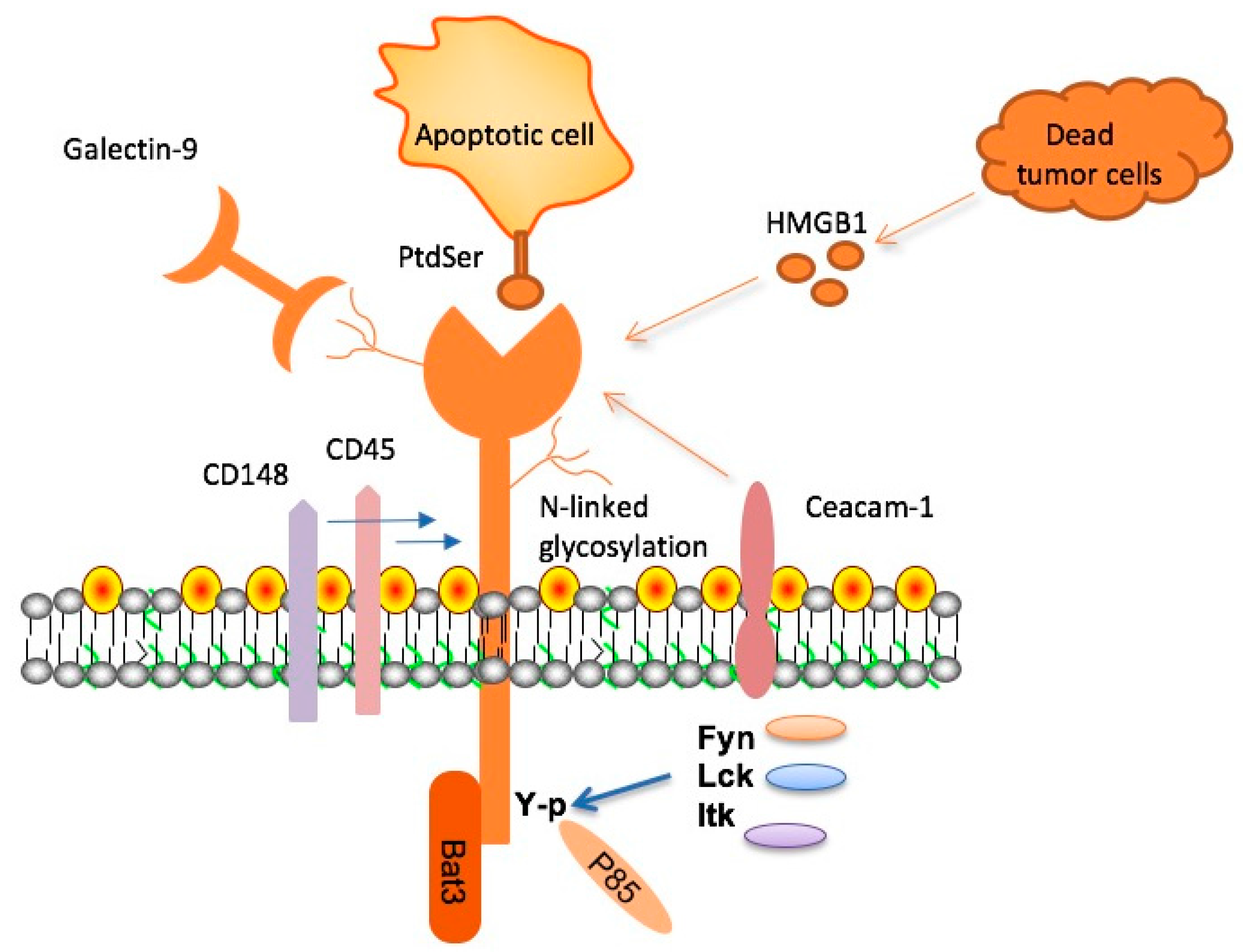
TIM-3 (T cell immunoglobulin and mucin domain 3) is an immune checkpoint molecule critically involved in regulating immune responses. This molecule has recently been highlighted as a key player in late-onset Alzheimer’s disease, which comprises approximately 90-95% of all Alzheimer’s cases. Research has shown that the genetic variants associated with TIM-3 are linked to increased risk for Alzheimer’s, suggesting a significant role in the disease’s pathology. By controlling microglial activity, TIM-3 can prevent these immune cells from effectively clearing toxic amyloid plaques, which accumulate in the brains of individuals with Alzheimer’s. This dysfunction may contribute directly to cognitive decline commonly observed in patients.
The effects of TIM-3 on cognitive function in Alzheimer’s are profound, as the overexpression of this molecule leads to a homeostatic state in microglia, inhibiting their ability to engage properly with harmful plaques. Findings from animal models indicate that the deletion of TIM-3 results in improved clearance of amyloid plaques, restoration of synaptic function, and partial recovery of cognitive abilities. These revelations highlight the potential of TIM-3 therapy for Alzheimer’s, presenting a novel approach to leverage immune mechanisms to combat neurodegeneration.
The Role of Microglia in Alzheimer’s Disease Pathogenesis
Microglia, the resident immune cells of the brain, are pivotal in maintaining neurological health. In Alzheimer’s disease, these cells become dysfunctional due to the accumulation of amyloid plaques and the inhibitory effects of molecules like TIM-3. Normally, microglia are responsible for synaptic pruning and clearance of cellular debris, which are essential for healthy cognitive function. However, when subjected to chronic activation induced by continuous plaque exposure, microglia can become ‘activated’ but may not efficiently clear these harmful aggregates, leading to detrimental outcomes such as inflammation and neuronal loss.
Research shows that a balance between microglial activation and inhibition is crucial for brain health. The persistence of TIM-3 expression results in microglial cells being stuck in a non-functional state, reluctant to engage in the phagocytosis of plaques. This underlines the importance of timing and regulation of immune responses in the brain; when microglial cells fail to clear amyloid beta, cognitive functions suffer. Addressing these dysfunctions through targeted therapies may not only alleviate symptoms but also halt the progression of Alzheimer’s disease.
Immune Checkpoint Therapy: A New Frontier in Alzheimer’s Treatment
Immune checkpoint therapy, which has revolutionized cancer treatment, is now being explored for Alzheimer’s disease, particularly through the modulation of TIM-3. This therapy aims to reverse the inhibitory signals that limit microglial function, thereby enhancing their ability to respond to amyloid plaques. By utilizing anti-TIM-3 antibodies or small molecule inhibitors, researchers aim to ‘release the brakes’ on microglial activity, allowing these immune cells to effectively clear accumulating toxic proteins in the brain.
Clinical trials for anti-TIM-3 therapies could represent a turning point in Alzheimer’s treatment strategies. Unlike traditional anti-amyloid therapies, which have had limited success due to issues with blood-brain barrier penetration and adverse risks, TIM-3 blockade offers a more refined approach. By specifically tuning the immune response and mitigating the risks associated with vascular damage, this method holds promise in enhancing cognitive function and potentially modifying disease progression.
Cognitive Function Restoration in Alzheimer’s Models
In studies involving genetically modified mice lacking TIM-3, researchers observed notable improvements in cognitive behavior and memory. These models allow for testing various therapeutic strategies by meticulously observing the relationship between microglial activity and cognitive outputs. The ability of these mice to navigate mazes and respond to stimuli demonstrates that therapeutic interventions targeting TIM-3 can successfully modify the course of cognitive decline associated with Alzheimer’s.
Such results underscore the importance of understanding cognitive functions in the context of Alzheimer’s pathology. By correcting the dysfunctional pathways that hinder microglial action, researchers have opened doors to innovative treatment avenues. Developing therapies that can facilitate microglia’s plaque clearance will be pivotal in restoring cognitive functions that are otherwise compromised in Alzheimer’s patients.
Genetic Links Between TIM-3 and Late-Onset Alzheimer’s
The relationship between TIM-3 and late-onset Alzheimer’s disease is underscored by genetic studies that have identified specific polymorphisms in the TIM-3 gene, HAVCR2. These genetic variations are not merely incidental; they correlate with increased TIM-3 expression on microglia in Alzheimer’s patients. This elevated expression may act as a barrier, preventing effective immune responses against amyloid beta plaques, which accumulate and contribute to the disease’s progression.
Understanding these genetic aspects highlights the need for personalized approaches in Alzheimer’s treatment. Recognizing individuals with high TIM-3 expression could lead to tailored therapies that target and inhibit this checkpoint molecule, enabling appropriate immune responses while minimizing adverse effects associated with sustained inflammation. This approach can transform the landscape of Alzheimer’s treatment, emphasizing the significance of genetic background in therapeutic outcomes.
The Therapeutic Potential of Anti-TIM-3 Antibodies
Therapeutic strategies involving anti-TIM-3 antibodies are becoming increasingly important in the fight against Alzheimer’s disease. These antibodies have demonstrated the potential to enhance the activity of microglia in clearing amyloid plaques by blocking inhibitory signaling. The success seen in preclinical models suggests that transitioning these findings into clinical trials could mark a pivotal advancement in Alzheimer’s therapy. By targeting TIM-3, these therapies may not only work to prevent cognitive decline but potentially recover lost functionalities.
While the development of anti-TIM-3 antibodies is still in its early stages, their selective targeting offers a promising alternative to traditional Alzheimer’s treatments. This innovative approach could minimize the risks associated with existing therapies and provide a more focused solution to the underlying problems caused by plaque accumulation. Embracing immune checkpoint therapy could rejuvenate research efforts in Alzheimer’s, sparking new hope for affected individuals and their families.
Current Challenges in Alzheimer’s Drug Development
The landscape of Alzheimer’s treatment has been fraught with challenges, particularly in developing effective drug therapies that yield meaningful cognitive improvements. Many traditional approaches focused on amyloid beta have failed, underscoring the complexity of Alzheimer’s pathology. The realization that targeting microglial function through immune checkpoint inhibitors like TIM-3 could be a viable pathway offers a fresh perspective amidst numerous setbacks in drug development.
Understanding these challenges involves not only exploring innovative therapeutic avenues but also enhancing the efficiency of clinical trials. The evidence surrounding TIM-3’s role in Alzheimer’s indicates that future drug development must embrace a more holistic view of immune system involvement in neurodegeneration. This comprehensive understanding will guide researchers in creating targeted therapies that can navigate the intricacies of Alzheimer’s disease and ultimately improve patient outcomes.
Future Directions for TIM-3 Research in Alzheimer’s
The future of TIM-3 research in Alzheimer’s is poised for exciting developments as researchers seek to translate findings from mouse models into human applications. Studies are currently underway to assess whether anti-TIM-3 therapies can prevent plaque accumulation in humanized mouse models. With rigorous investigation into the efficacy and safety of these therapies, there is potential for significant breakthroughs in addressing Alzheimer’s pathology.
As researchers identify mechanisms through which TIM-3 mediates microglial function, the focus will likely expand beyond plaque clearance. Investigating the broader implications of TIM-3 in neuroinflammation and cognitive health could refine strategies aimed at not just treating, but potentially preventing Alzheimer’s disease. This pioneering research may illuminate transformative pathways to develop novel therapeutic options that prioritize cognitive preservation and enhance quality of life for individuals with Alzheimer’s.
Integrating TIM-3 Therapy with Existing Alzheimer’s Treatments
As the understanding of TIM-3 in Alzheimer’s deepens, integrating TIM-3 therapy with existing treatment strategies could yield a multifaceted approach to management. Combining immune checkpoint therapy with current methods targeting amyloid beta and tau proteins might enhance overall efficacy, potentially leading to more comprehensive treatment outcomes. This integrative strategy can leverage synergistic effects, creating a holistic approach to tackling Alzheimer’s disease.
Additionally, prescriptive therapy based on TIM-3 modulation can involve comprehensive patient assessments for personalized medicine strategies. By monitoring genetic backgrounds, risk factors, and response to treatments, healthcare providers can tailor interventions that maximize the benefits of TIM-3 blockade alongside conventional Alzheimer’s therapies. This dynamic approach could redefine how practitioners manage Alzheimer’s disease, promoting better patient care and improved quality of life.
Frequently Asked Questions
What is TIM-3 therapy for Alzheimer’s and how does it work?
TIM-3 therapy for Alzheimer’s focuses on inhibiting the TIM-3 molecule, which prevents microglial cells in the brain from clearing amyloid plaques. By blocking TIM-3, microglia can attack and remove these harmful plaques, potentially improving cognitive function in individuals with Alzheimer’s disease.
How does TIM-3 relate to immune checkpoint therapy in Alzheimer’s treatment?
TIM-3 is an immune checkpoint molecule that regulates the immune response. In Alzheimer’s treatment, targeting TIM-3 can release microglia from their inhibited state, allowing them to effectively clear amyloid plaques and improve cognitive function, similar to how immune checkpoint therapy is used in cancer treatment.
What role do microglia play in Alzheimer’s and how does TIM-3 affect them?
Microglia serve as the brain’s immune cells, responsible for clearing out waste, including amyloid plaques associated with Alzheimer’s. TIM-3 inhibits microglial activity, preventing them from attacking these plaques. By blocking TIM-3, researchers aim to enhance microglial clearance of amyloid beta, improving cognitive function.
Are recent studies showing the potential of TIM-3 therapy for Alzheimer’s cognitive function?
Yes, recent studies indicate that TIM-3 therapy could enhance cognitive function in Alzheimer’s models by enabling microglia to clear plaques more effectively. Research has shown improved memory and cognitive behavior in mice with deleted TIM-3, suggesting a promising avenue for Alzheimer’s treatment.
What is the significance of the TIM-3 gene in Alzheimer’s disease?
The TIM-3 gene, linked to late-onset Alzheimer’s disease, acts as a genetic risk factor. Variants of this gene result in increased TIM-3 expression on microglia, which stops them from clearing harmful amyloid plaques, contributing to Alzheimer’s progression. Targeting TIM-3 may mitigate these effects.
What are the prospects of anti-TIM-3 antibodies in Alzheimer’s treatment?
Anti-TIM-3 antibodies show promise in Alzheimer’s treatment by targeting the TIM-3 pathway to boost microglial function in plaque clearance. Current research aims to determine the efficacy of these antibodies in preventing plaque accumulation, which is crucial for developing effective Alzheimer’s therapies.
How does TIM-3 therapy differ from traditional Alzheimer’s treatments?
TIM-3 therapy differs from traditional Alzheimer’s treatments by focusing on modulating the immune response rather than solely targeting amyloid plaques with direct drug interventions. This innovative approach aims to enhance the brain’s natural clearance mechanisms by boosting microglial activity.
What are the potential side effects of targeting TIM-3 in Alzheimer’s therapy?
While targeting TIM-3 may improve cognitive function by enhancing microglial activity, potential side effects could arise from altering the immune response, including the possibility of increased inflammation or autoimmune reactions. Ongoing research is crucial to evaluate the safety and efficacy of this approach.
When can we expect TIM-3 therapy for Alzheimer’s to become available for patients?
The timeline for TIM-3 therapy for Alzheimer’s is uncertain as research is still in preclinical stages. Future clinical trials will need to demonstrate safety and efficacy in humans before any potential treatment can become available, which may take several years.
What does the future hold for TIM-3 research in Alzheimer’s treatment?
The future of TIM-3 research in Alzheimer’s treatment is promising, with ongoing studies examining its role in plaque clearance and cognitive function. Continued advancements in this area may lead to innovative therapies that significantly improve outcomes for individuals with Alzheimer’s disease.
| Key Points |
|---|
| TIM-3 therapy for Alzheimer’s leverages a strategy previously effective in cancer treatments. |
| TIM-3 is a checkpoint molecule that inhibits microglia, the brain’s immune cells, from clearing amyloid plaques associated with Alzheimer’s. |
| The study shows that deleting TIM-3 in mice improved their ability to clear plaques and restored some cognitive function. |
| Research indicates that TIM-3 might be a genetic risk factor for late-onset Alzheimer’s, which represents 90-95% of cases. |
| Blocking TIM-3 with specific antibodies could potentially enhance microglia’s ability to clear plaques in Alzheimer’s patients. |
| Future therapies could emerge from existing anti-TIM-3 antibodies, repurposed for Alzheimer’s treatment. |
Summary
TIM-3 therapy for Alzheimer’s represents a promising breakthrough in the quest to combat this debilitating disease. Researchers have discovered that targeting the TIM-3 molecule, which inhibits microglial function, can enhance the brain’s ability to clear harmful amyloid plaques. By deleting the TIM-3 gene in mice, scientists observed significant cognitive improvements, suggesting a potential pathway for restoring memory function in Alzheimer’s patients. With ongoing studies into the use of anti-TIM-3 antibodies, the future of Alzheimer’s treatment may be brighter, aiming to transform ineffective approaches into effective strategies that could change the lives of millions.